At home, laundry is about removing grease, grass, and coffee stains without damaging the fabric of the clothing. Energy and water efficiency only matter in a secondary sense. Rinse water is plentiful, and the only water quality consideration is that it is sufficiently soft to avoid interference with the detergent and calcium build-up in the clothes.
For the cruiser, however, the fresh water we need for rinsing is like gold. If we follow the rough proportions we use at home, its easy to go though more than a gallon per garment, or about 12-16 gallons per day for a crew of four. Unless you have a high-volume water maker, that’s going to put a serious dent in your water tankage. Add drinking, cooking, and even minimal bathing, and you’ll be looking for water constantly. This can work for marina hoppers, and we’ve used a lot of laundromats, but what if you want to stay on the hook longer or make passages?
If we wash in freshwater, can we eliminate rinsing entirely by using ammonia instead of detergent? If we wash in seawater, can we minimize the rinsing through the use of rinse aides or vinegar? These are just a few of the questions readers posted.
What We Tested.
We tested a high efficiency (HE) laundry detergent (Arm and Hammer Clean Burst) and ammonia, washing in fresh or seawater using several water-conserving rinse alternatives.
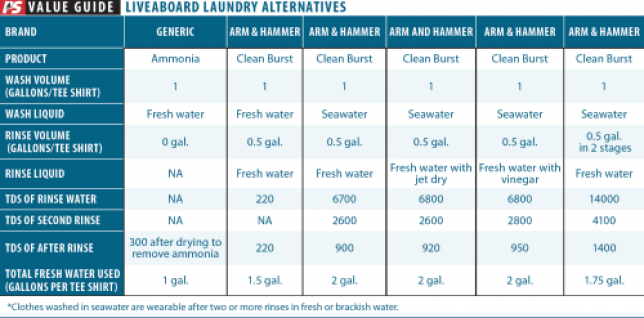
How We Tested
We set our laundry talents to work on a collection of identical 100 percent cotton work T-shirts with reasonably identical soil. We washed using gallon of water per T-shirt and rinsed with gallon of water. After washing, each garment was spun dry for uniform drying, roughly equivalent to a good hand wringing. We used an O-Cedar EasyWring bucket to provide uniform washing and wringing. The table documents the exact volume of wash and rinse water and the amount of cleaning aid used in each trial (see table page 19). Residual salt was measured using conductivity (TDS meter). We also graded comfort qualitatively.
Observations
Ammonia. The advantage of ammonia is that it is a cleaning agent that will evaporate, eliminating the need for a rinse to remove the soap. The ammonium ion is somewhat effective in emulsifying grease and breaking down proteins that form sweat stains. After the clothing dries in the breeze, the ammonia is long gone. It yielded the softest, most comfortable T-shirts of any reduced-water laundry method tested. Ammonia is the clear winner when water is scarce.
The downsides are that rinsing also carries the dirt away, so with reduced water volume comes reduced cleaning. Since wool and silk are proteins, they must never be washed with ammonia. Used at home, ammonia can also be hard on the washing machine. Altogether, there are reasons ammonia is not the ultimate laundry product.
Vinegar. Folklore says that vinegar helps remove salt from clothes. We couldn’t see the logic in this for removing sodium chloride, the very soluble salt we think of as sea salt. In fact, we found it had no effect, even after we corrected for conductivity attributable to the vinegar left in solution.
But this may not be the whole story. Each time you do laundry with seawater, the sodium salts will rinse out, but the calcium salts will tend to build up. Since seawater is nearly saturated in calcium it is not effective at removing it during washing. The clothes (and the lines on our boat) get stiffer over time, as calcium builds up, and thus a long vinegar rinse every third laundry or so will remove the calcium. So the homespun lore regarding vinegar is half true.
Vinegar is not compatible with many washing machine hose and seal materials, including natural rubber, buna, nitrile, and silicon (EPDM is good). This is not because it is acidic, but because the organic portion (similar to drinking alcohol), like a solvent, is aggressive to many elastomers. Such organic materials are well known to be a problem at very low levels in engine coolants, for example, and are restricted by specifications to 10-50 ppm levels.
Rinse Aides. We tried Jet Dry and Cascade rinse aides. It had been suggested clothes would drip or wring drier. No difference.
Detergent. First, it seems logical to use an HE detergent; they are designed to use less. Second, unless you have been snuggled up next to the engine, you can probably use considerably less than the manufacturer suggests. In fact, we generally cut it back for home laundry, unless weve been up to something messy, in which case I might increase the detergent, use hot water, and wash twice!
Seawater or fresh. Every wash-wring-rinse regimen is different and garments vary in their tendency to hold onto water. Our test procedure dramatically reduced the salt content in 100-percent cotton T-shirts. This can be improved by using a wringer.
Multiple stage rinse.This is common practice in the chemical industry. The rinse water can be used repeatedly, in stages though 2-3 buckets of progressively fresher water, adding fresh water to the last bucket as needed. Laborious, but it reduces the required amount of water by about 30%.

Brackish water. Coastal sailors often find themselves anchored in water with salinity that is 2-4 times lower than seawater. Assuming the water is relatively clean (there is often some algae and silt, but it will come out in the rinse) the required amount of rinse water will be proportionately less, comparable to seawater laundry that has been rinsed one more time.
Salt from sweat. Laundry water isn’t the only source of salt in our clothes. We sweat into them in warm weather, and the sweat dries. Although the composition varies with the person and acclimatization, it is typically about 1000 ppm salt and we can expect to sweat out 2 quarts in warm weather (additional water is lost breathing). A wrung-out T-shirt holds about one pint of water, so that is equivalent to about 400 ppm TDS rinse water, or a shirt that has been washed in seawater and rinsed three times. Just a comparison point.
Pick your clothes. Although no fabric magically rejects salt, quick drying synthetics hold a lot less water when laundered. A cotton T-shirt feels wonderful when first put on, but will it feel as good once it gets sweaty? You can launder 3-5 pairs of fleece pants or jackets in the same amount of water required for one heavy cotton equivalent. Many folks worry about how clothes pack or what they weigh. If you are thinking about laundry, think about what they weigh wet and how they wring out (socks and underwear are exceptions).





































Very impressive article thanks to the scientific methodology!
I can’t follow the article. Missing is how much of any cleaning agent is used—how much ammonia or Burst?
Missing is a conclusion/best practice—is ammonia the winner or not? The negatives seem quite bad. And as asked above, how much ammonia are we bringing on board for, say, 2-3 washes for the crew?
How are washes done? Hand agitating?, soaking?, is the O-Cedar involved or not, or are you recommending it be used just for wringing or are you saying a good hand wringing is better (as in, don’t waste space having any special equipment)?
Aren’t these questions worth having answers for? Thanks
I agree. More instructional and directional content would be good here. It feels like the article ended abruptly without making useful recommendations or overall conclusions.
Agreed. Also, labor is just as big a factor as fresh water useage. What about “smart wool” merino wool type garments? These require less laundering due to their natural antibacterial properties. Amonia is a harsh, smelly liquid as well, and difficult to source in large quantities due to it’s use in methamphetamine manufacturing. There are better alternatives
We need more details. Especially helpful would be a “conclusions” paragraph to sum it all up.
Sorry to pile on, but a few ideas tossed around without solid conclusions only begs more questions. Trust me this has become a universal problem with so much space to fill on webpages versus the limited amount of column inches in a traditional magazine format. Necessity has given us another level of so-called “journalism”. I call it the space-filler writer.
This article was restructured in the “fitting” process. The conclusion, as well as some other bits, didn’t make the cut.
Conclusions. Seawater laundry was less impractical than we expected. It does put a premium on tight wringing at each step, and doing enough laundry at one time so that rinse water can be recycled through multiple rinses is a big help. When using all freshwater, vinegar makes a legitimate rinse-aid, this coming from a person who never uses vinegar on a salad. Ammonia didn’t impress us; a freshwater rinse is still required to avoid scratchy clothes, in which case we’d rather just use a pinch of conventional detergent.
The bucket press drying methods has less potential to damage clothes than hand wringing, but does not get them nearly as dry. We’d opt for careful hand wringing for the occasional emergency laundry requirement; nothing more to carry. If we were going to carry something, we’d opt for a spinner; it used the least water, was safe for the clothes, and let us work through a pile of laundry with minimal effort. If we were cruising full-time, we’d make room for a handcrank ringer, like grandma used and like car washes still use for drying chamois; bulky and heavy, but easy-to-use, easy on the clothes, and effective.
In the past I experimented with washing on board. I tried soap and ammonia – I think you did better with vinegar than me. I used a large bucket with a fitting lid and roped it on, then sat it in the bows for the day underway. Used about 4 litres to wash a few things. Similar to rinse. The mop squeeze bucket is a v good idea. But the real problem was drying – unless you could anchor in a land breeze things just stayed a bit salty.
An alternative is to throw your laundry overboard so it can be washed ashore. rofl ; )
It depends where you sail as to what clothes you wear and what works in a ‘comfortable’ climate will be less applicable in higher Northern or Southern latitudes. But washing underwear and the occasional ‘T’ short is really not a big issue – anywhere. Sheets and towels are the big problem.
One answer is to carry plenty of spares, accumulate the dirty bed and bath linen and plan to reach a laundromat. If you do this then simply take extra underwear – and do it all at once. After trying various options this works best for us for 3 months, or so, off piste