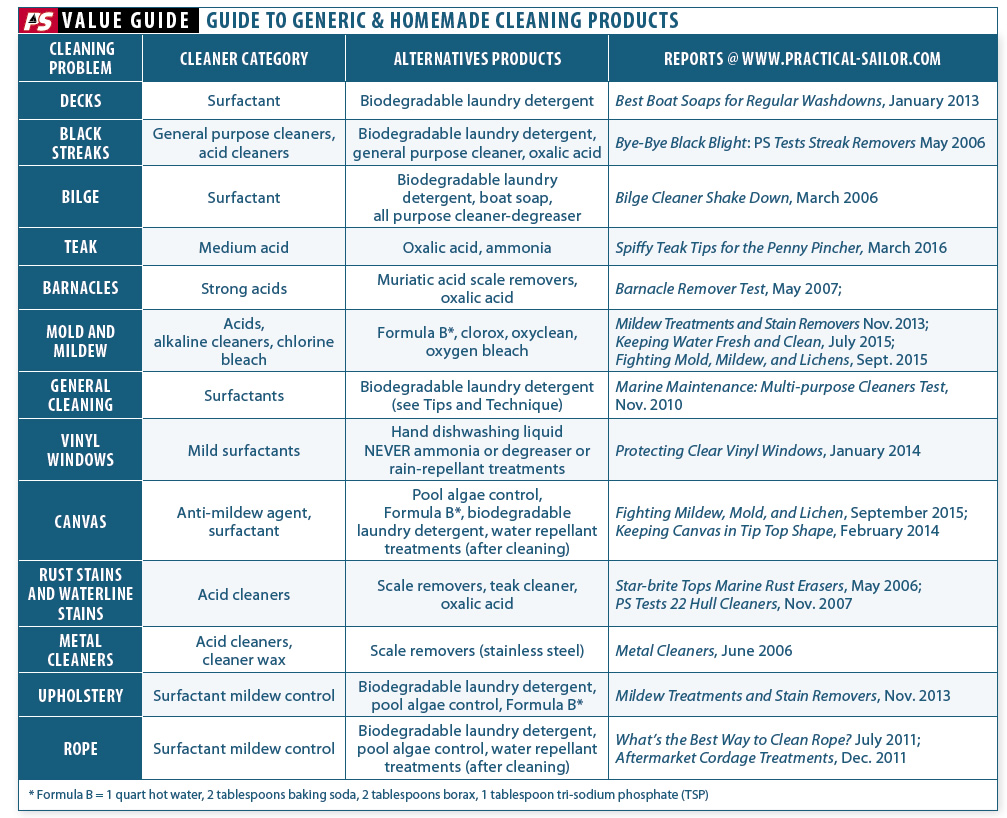
Bright, shiny and new looking. That was what we wanted when were shopping for our dream boat, and that is the impression our new-used PDQ catamaran gave. What was behind that spit-shine on a used boat? Besides the obvious hours of labor by the previous owner, it took two baskets of cleaning products, all stuffed in the stern lockers.
I needed the space back. The previous owners were a retired couple, and I would be cruising with kids. The stern lockers were for their stuff. I also knew that a showroom shine was not in my near future, and that my daily cleaning needs would be more basic. It was all good usable stuff-we took it all home, and weve been living off this horde for years. But the duplication and overlaps in function and chemistry were ridiculous, and there was no pattern behind the selections that we could see.
Glancing at the products with a chemists eyes, we pared down dozens of specialty products to just a few products capable of same range of cleaning, which easily fit in a small pail.
The Basics
Years ago, I challenged a chemical salesman over the price of some industrial cleaners he offered. Packaged in 55-gallon drums, rather than consumer spray bottle packaging, they struck me as overpriced for what they contained. We price chemicals based on what they can do for you, not what they cost, he replied. And that is foundation of plastic-bottle cleaner game; create a pleasing package, a catchy name, and focus on what it can do-not whats in the bottle. Distract your customers from the fact that the $16 spray bottle contains only 16 cents worth of stuff.
If you dig past the picture and top-line claims and read the ingredients, you can learn the real story, or at least understand what youre buying and have some understanding of how it works.
If you try to sort cleaners according to each particular task, as the marketers would like us to do, you have almost endless permutations. These products are sorted first by the material they are supposed to clean (fiberglass, carpet, clear vinyl, etc.) and then are sorted by the type of soil or stain (and grease, metal stains, mildew, etc.).
A more efficient approach for the cruising sailor with limited budget and space is to sort them by what they are: acids, bases, surfactants, oxidizers, solvents, or emulsions. Instead of requiring 30 random products, you would need only four or five with carefully targeted chemistry. Thats it-no more than five cleaners to serve most purposes, saving money, and more importantly space and weight. The challenge-and it is a small one-is to know the nature of the stain.
Do no harm. First, we must not damage what we are cleaning. Harsh chemicals are often employed to do the heavy lifting, and we don’t want them eating up our project. When in doubt, test on a scrap of the same material or in an inconspicuous location.
Strong oxidizers. Bleach, hydrogen peroxide, cleaners containers peroxides, percarbonates, and hypochlorites fall into this category. They can bleach and weaken fabrics. If properly diluted and exposure time is limited, oxidizers are generally safe for materials that are non-porous or color-fast in the sun.
Solvent-containing products. Solvents can be tough on certain plastics. Degreasers often contain citrus oils or glycol ethers (ethylene glycol monobutyl ether is a common co-solvent), which can damage some paints and plastics. Formula 409, Spray Nine, and anything that has orange in the name is suspect. Clear soft vinyl windows require special precautions (Ultimate Guide to Caring for Clear Plastic, Practical Sailor, July 2014).
Acids. Muriatic acid (3 percent solution, diluted 10:1) is quite useful for removing calcium or rust stains. However, the pH is very low and it will quickly corrode aluminum and most other metals. Nylon can be severely damaged and even melted by strong acids (a few drops of battery acid on a loose thread is one way to identify nylon). Milder acid products, such as vinegar (acetic acid), lemon juice (citric acid), and CLR (lactic acid) are both slower and safer to work with.
Alkalis. Lye (sodium hydroxide) is found in drain cleaners, paint removers, and oven cleaners. It is very effective on cooked-on grease proteins and fats, but the very high pH that makes it effective can corrode aluminum and brass and remove or dull paint. The more moderate alkalinity of washing soda (sodium carbonate) or baking soda (sodium bicarbonate) are sometimes more appropriate.
When using either acids or alkalis, remember the following:
Protect your work. Many cleaners can streak or corrode if used incorrectly:
Protect what you don’t want to contact. If preventing contact is impractical, wet it down; the chemical will be diluted, which is often enough to prevent harm.
Dont let it dry. Bleach, for example, while normally harmless to most surfaces, becomes very alkaline (it contains lye) when allowed to dry, and can eat little pits in aluminum and etch glass. It wont do this if you keep it wet.
Clean from the bottom up. This may seem counter intuitive, but it is related to don’t let it dry. If you clean from the top down, undiluted cleaner will streak down the sides, resulting in uneven cleaning and etching. Your final cleaning and rinse can be top-down.
Rinse completely. The aim is to remove any residue. Use a shop-vac or carpet extractor if cleaning carpet or upholstery. Consider using a weak anti-mildew treatment in the rinse step. Dont go from dirty, to clean, to smelly because it didnt dry and you left biodegradable soap (mildew food) in it.

Cleaning Chemistry 101
There are four general approaches to cleaning, often with several combined in one product, though some are mutually exclusive (either opposites, or they react with each other).
Low pH
By definition, chemicals with low pH are acids. In rough order of aggressiveness, the most common ones we use are: muriatic (hydrochloric), oxalic (teak cleaners), citric (lemon juice), lactic (CLR), acetic acid (vinegar). Some of these products contain surfactants. They work well on the following:
- Metal stains. These include rust (iron III hydroxide), aluminum black streaks (aluminum II oxide), water spots (salt and calcium hydroxide).
- Soap scum.
- Waterline stains, including tannin stains.
- Uncured epoxy. Organic acids, such as vinegar, react with unreacted monomer, stopping the curing process and softening uncured epoxy. Much better than soap or solvent.
High pH
These are the alkalis, in rough order of aggressiveness: sodium hydroxide (lye, drain cleaners, paint strippers), ammonia, washing soda, baking soda). They are generally combined with other surfactants to clean the following:
- Heavy grease, particularly if cooked-on. The high pH hydrolyzes the soap (acid can also be effective-corrosiveness to metals depends on the metal, so test first).
- Removing or de-glossing many paints. This is either handy (Easy-Off is a handy paint and varnish remover for small spots) or disastrous. It is also very hard on skin (dissolves the fats that make up the cell walls).
Solvent and co-solvents
These include D-limonene, citrus oils, ethylene glycol monobutyl ether (Celusolve), and petroleum spirits). Usually, these solvents are combined with other surfactants to clean the following:
- Oil and grease. Solvents have much of the grease-cutting ability of alkaline cleaners, but without the high pH. They can harm plastics and some paints. Not generally as effective as alkaline cleaners on fats, but gentler on skin and many surfaces.
Oxidizers
These include bleaching agents, in rough order of aggressiveness: sodium hypochlorite, hydrogen peroxide, calcium percarbonate. The effectiveness is influenced by pH; mixing with baking soda (a few teaspoons per quart) moderates the pH of stronger hypochlorite solutions, increasing bleaching and disinfection effectiveness dramatically. Never mix bleach with acids or ammonia, which will create a toxic gas. Oxidizers are effective on the following:
- Organic stains (mildew). Bleaching metal stains and tannin stains will often only set them, making them more resistant to subsequent treatments (you are trying to oxidize an oxide, which wont work).
Dont mix with organic cleaners (most surfactants and degreasers). Bleach reacts with many organic ingredients, destroying both. Bleaches can be mixed with borax, TSP, and baking soda to control pH.
Bleach eliminates stains because it breaks apart bonds within chromophores in molecules, the structures responsible for color. The compounds that cause the stain may become colorless, but may still exist, explaining why stains often return quickly.
Many cleaners, specifically acids, caustics, and oxidizers, work better by prolonging the soak time. However, unless you can put the article in a bucket, this can be a little awkward. For bleach, making a paste with baking soda is time-proven, holding the bleach in place and buffering the pH. For acids and caustics, soaking it in a rag can work, although the rag may be damaged in the process. A final trick is to mix the cleaner with an inert powder; cabosil/fumed silica works very well for nearly all chemicals, stiffening them without affecting the chemistry.
Conclusion
Hopefully this report, prompted a little more consideration regarding the sort of stain you are actually removing, will help lead you to the best cleaner for each job. Being able to narrow down your cleaning supplies to a few essential products is not so critical for the marina-bound boat, but its becomes more important once you begin a long cruise.
All we carry while cruising is hand dishwashing soap, laundry detergent, vinyl window cleaner (Star Brite View Guard), an acid-based cleaner (CLR), and anti-mildew agent (do-it-yourself Formula B), and a degreaser (Star Brite Degreaser or Sudbury Bilge Cleaner). At the boatyard we focus additional products on specific needs; a protectant for the vinyl windows (IMAR 302), a vinyl treatment (303 Aerospace), generic oxalic acid for most heavy duty scale and metal stain removal needs, and a paste wax (Meguiars Cleaner Wax) for most everything else. Some of our applications are off-label, like Easy-Off as a paint remover, or CLR for the waterline stain. But having satisfied all of our marine cleaning needs with just a few versatile products, we now have an answer for every problem and a lot more space in our lockers.
All that said, there are some very effective specialty formulated marine products that might outperform our basic kit. If you are a stickler for a clean boat, want to impress guests (or potential buyers), or are having trouble with tough stains, check out our four-volume Ebook series: Marine Cleaners, the Complete Series, which covers more than you need to know to keep your boat looking her looking best.
Drew Frye is a frequent contributor to Practical Sailor. He sails a 32-foot PDQ catamaran on the Chesapeake and is author of the Delmarva Cruising Guide. His website is www.delmarva.com.