In the July 2013 issue of Practical Sailor, contributor Drew Frye plunged into the not-so-funny topic of joker valves (if you don’t know what this is yet, consider yourself lucky) and emerges with some valuable tips on keeping our marine heads healthy. One of his potentially controversial discoveries is that the “eco-friendly” anti-freeze propylene glycol isn’t any kinder to the marine environment than the anti-freeze it was designed to replace – ethylene glycol – and it is definitely harder on plumbing components.
Granted, neither of these antifreeze products are beneficial for the environment, and we should do our best to minimize their use and participate in recycling programs. On land, relatively small doses of ethylene glycol-the product that seems to be the least harmful to our marine heads-is lethal to mammals. But in a marine environment, neither glycol packs the same punch. I wont dive into the reasons for this, nor elaborate on the ecological arguments in favor of ethylene glycol here. There are some links below, so you can see for yourself why we think ethylene glycol has been unfairly tarred with regards to marine use.
Here, I’ll focus only on the effect that these chemicals have on the neoprene and nylon components in our engines and plumbing-including joker valves. For those who are still wondering what a joker valve is, it is a nitrile or neoprene valve in your marine toilet that prevents flushed material from flowing back into the bowl. It is also one of the most common failure points in marine plumbing. Most head manufacturers recommend replacing it every one or two years. (See the adjacent photo of a Raritan joker valve.)
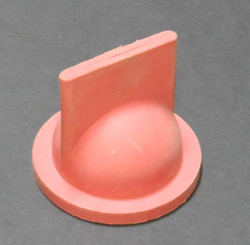
From a practical standpoint, the problem is clear: Propylene glycol is harmful to neoprene, a material commonly used in valves, seals, and impellers. Ethylene glycol is not as harmful. While neoprene does not fully degrade in propylene, it does change size and distort a bit, potentially causing a joker valve leak. It’s not just joker valves that are at risk. We were told by several experienced marine mechanics that the best way to ruin the engine raw water-pump impeller is to winterize with propylene glycol without removing the impeller.
Probably the clearest example of what propylene glycol can do to marine plastics is what we found in our test of nylon strainers. In addition to joker valves, Frye tested nylon strainers (Shurflo) found in many potable water systems. One pair was exposed to glycols in the laboratory, and one pair was tested in use on a test boat.
The result in the lab was troubling; the strainer exposed to propylene glycol was severely crazed and ruined, and the strainer exposed to ethylene glycol was slightly crazed. The pair exposed on the boat showed even more damage (pictured at top). We think this was because temperature changes on board were more drastic than in the lab and because of the mechanical strain on the threads. The cracks caused by ethylene glycol were not visible for several days and were nearly overlooked, as glycol in the cracks rendered them invisible; only after soaking the strainers in water and allowing them to dry for several days did the cracks appear. Thus, when inspecting strainers, wash and dry them before checking for cracks. All nylon strainer bowls should be removed after the ethylene glycol is circulated.
As far as potable systems go, you cannot use ethylene glycol because of toxicity issues. Rather than put safe propylene glycol in the tank, perhaps a better option is to drain the tank, and empty low spots in the plumbing-although that is not always possible.
To find out which joker valve performed best in our tests, and to learn which common head cleaning and lubricating products actually do more harm than good, check out the article in the July 2013 issue of Practical Sailor.
And for more important tips on the maintenance of your engine, and cruising in general, check out Beth Leonard’s The Voyager’s Handbook, 2nd edition.
Resources
Conference abstract discussing ethylene glycol vs. propylene glycol toxicity
EPA review of ethylene glycol and propylene glycol aircraft deicer.
Material Safety Data Sheet for ethylene glycol
Material Safety Data Sheet for propylene glycol
Comparison of life cycle impact of propylene glycol vs. ethylene glycol





































